More rat-related grants are good news for all of us so we’re assembling some information and other resources that might be of help as you plan and write your grants. This is certainly a work in progress so please feel free to contact us with more suggestions and requests for ways in which RGD can help you in your grant writing.
Rat Data and Statistics
The RGD Curation team has a great deal of experience finding and compiling rat data and we may be able to help you find information or even create specific datasets for your grant or publication. If you need statistics we encourage you to contact us.
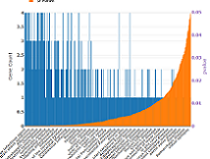
Current Rat data statistics from NCBI
You can search across all NCBI databases using the term “rattus norvegicus” to see the number of rat records in each database and access the corresponding lists of records: [Run this query at NCBI now].
Data Sharing Plan
RGD makes data readily available to users through our website and tools, as well as tool-specific download functions, our Download page and our Rest APIs. We are happy to work with investigators looking for a particular dataset, and groups building data pipelines for their own purposes, to supply customized data sets and to add new data files to our FTP site or new APIs to our list. Contact us for more information or if you need a particular set of data.
RGD is also a founding member of the Alliance of Genome Resources (https://www.alliancegenome.org. We submit data regularly to the Alliance for integration into their database with data from other model organisms such as yeast, zebrafish, worm, fly and mouse. In addition to providing an additional outlet for data sharing, the Alliance facilitates cross-species comparisons that could be of use to researchers looking to present their work in a larger context.
Most NIH grants now contain a section covering the Sharing of Research Data (eg. RO1, Section 2.C.) which requires applicants to describe their plans for sharing data that is created as part of the proposed research. RGD is well positioned to act as a suitable venue for project data to be made available to the research community and we encourage researchers to contact us with any questions they may have on how to do this. Strain, QTL, and gene/allele registration can be done through our submission forms.
Direct submission of new large datasets, or additions and revisions to existing data in RGD are welcome. These would include quantitative phenotype data (i.e. measurement values from low-throughput and high-throughput phenotyping projects), strain-specific variant data from whole genome/exome sequencing, and RNA-Seq data. Please contact our data manager Jennifer Smith for these types of data submissions.
If you need a Letter of Support (LOS) for your grant stating that RGD will make your data publicly available, please do not hesitate to contact us to request a LOS.
Software Sharing Plan
Where third party software exists that meets a specific need at RGD, we use that software. Examples of this include our use of JBrowse (https://jbrowse.org/) for our genome browsers, InterMine software (http://intermine.org/) for RatMine and Cytoscape.js (http://js.cytoscape.org/) for our InterViewer display. Information about these tools and how to obtain source code for them can be found on the respective websites for the software. For information about RGD’s specific implementations of these tools, please contact us.
Where we have been unable to find software that meets a specific need at RGD, we have developed custom software and tools to support our needs and those of our users. RGD source code is hosted at GitHub (https://github.com/rat-genome-database) and can be used, modified, and distributed under the terms of the GNU Public License version 3. (GPL-3.0).
Model Organism Sharing Plan
Most NIH grants now also contain a section covering the Sharing of Research Resources (e.g., RO1, Section 2.D.) which requires applicants to describe their plans for sharing “unique research resources” that are created as part of the proposed research (see NIH Grants Policy Statement for more information). This includes a Model Organism Sharing Plan that should describe how unique animal models created through the funded research will be made available to the community.
While RGD is unable to help directly with the sharing (i.e. maintenance, preservation and/or distribution) of biological resources such as the rats or other model organisms, investigators are encouraged to contact groups such as the Rat Resource and Research Center (RRRC) in Missouri, USA or the National BioResource Project for the Rat (NBRP-Rat) in Kyoto, Japan, for information about their rat strain repository facilities. We do recommend, however, that you do contact us for correct nomenclature of your strain before submission of the rats to either RRRC or NBRP. The use of official nomenclature and unique identifiers for your biological resources is required for following the “FAIR” principles and for helping to make your research reproducible.
Weekly Funding Opportunities and Policy Notices from the National Institutes of Health.
- Notice of NIAAA Participation in PAR-18-331 “Simulation Modeling and Systems Science to Address Health Disparities (R01 Clinical Trial Not Allowed)” – Notice NOT-AA-17-015 from the NIH Guide for Grants and Contracts
- Notice of Change in Participant Eligibility for PAR-17-051 “Postbaccalaureate Research Education Program (PREP) (R25)” – Notice NOT-GM-18-007 from the NIH Guide for Grants and Contracts
- Notice of Intent to Publish a Funding Opportunity Announcement for National Centers for Translational Research in Reproduction and Infertility (P50) – Notice NOT-HD-17-024 from the NIH Guide for Grants and Contracts
- Pathogenesis of Age-Related HIV Neurodegeneration (R01 Clinical Trial Not Allowed) – Funding Opportunity RFA-AG-18-023 from the NIH Guide for Grants and Contracts. NeuroHIV is at an inflection point, with an urgent need to understand the mechanisms that cause and modulate the CNS impairment in the era of antiretroviral therapies.
- The NCI Predoctoral to Postdoctoral Fellow Transition Award (F99/K00) – Funding Opportunity RFA-CA-18-001 from the NIH Guide for Grants and Contracts. The purpose of the NCI Predoctoral to Postdoctoral Fellow Transition Award (F99/K00) is to encourage and retain outstanding graduate students recognized by their institutions for their high potential and strong interest in pursuing careers as independent cancer researchers. The award will facilitate the transition of talented graduate students into successful cancer research postdoctoral appointments, and provide opportunities for career development activities relevant to their long-term career goals of becoming independent cancer researchers. This Funding Opportunity Announcement (FOA) does not allow applicants to propose to lead an independent clinical trial, but does allow applicants to propose research experience in a clinical trial led by a sponsor or co-sponsor.
- Clinical Trials to Test the Effectiveness of Treatment, Preventive, and Services Interventions (Collaborative R01-Clinical Trial Required) – Funding Opportunity RFA-MH-18-700 from the NIH Guide for Grants and Contracts. This Funding Opportunity Announcement (FOA) seeks to support clinical trials to establish the effectiveness of interventions and to test hypotheses regarding moderators, mediators, and mechanisms of action of these interventions. This FOA supports clinical trials designed to test the therapeutic value of treatment and preventive interventions for which there is already evidence of efficacy, for use in community and practice settings. Applications might include research to evaluate the effectiveness or increase the clinical impact of pharmacologic, somatic, psychosocial (psychotherapeutic, behavioral), device-based, rehabilitative and combination interventions to prevent or treat mental illness. This FOA also supports clinical trials to test patient-, provider-, organizational-, or systems-level services interventions to improve access, continuity, quality, equity, and/or value of services. The intervention research covered under this announcement is explicitly focused on practice-relevant questions.
- Clinical Trials to Test the Effectiveness of Treatment, Preventive, and Services Interventions (R01- Clinical Trial Required) – Funding Opportunity RFA-MH-18-701 from the NIH Guide for Grants and Contracts. This Funding Opportunity Announcement (FOA) seeks to support clinical trials to establish the effectiveness of interventions and to test hypotheses regarding moderators, mediators, and mechanisms of action of these interventions. This FOA supports clinical trials designed to test the therapeutic value of treatment and preventive interventions for which there is already evidence of efficacy, for use in community and practice settings. Applications might include research to evaluate the effectiveness or increase the clinical impact of pharmacologic, somatic, psychosocial (psychotherapeutic, behavioral), device-based, rehabilitative and combination interventions to prevent or treat mental illness. This FOA also supports clinical trials to test patient-, provider-, organizational-, or systems-level services interventions to improve access, continuity, quality, equity, and/or value of services. The intervention research covered under this announcement is explicitly focused on practice-relevant questions.
- Early Stage Testing of Pharmacologic or Device-based Interventions for the Treatment of Mental Disorders (R61/R33-Clinical Trial Required) – Funding Opportunity RFA-MH-18-702 from the NIH Guide for Grants and Contracts. The purpose of this Funding Opportunity Announcement (FOA) is to support the early stage testing of pharmacologic interventions with novel mechanisms of action, or device-based interventions, for the treatment of symptoms or domains of altered functions in individuals with mental illness (e.g., schizophrenia, depression, autism, obsessive compulsive disorder, anxiety, bipolar disorder). Early intervention studies are also encouraged where symptoms of a disorder have been identified in subjects (a prodromal phase), prior to full diagnostic criteria being met. Ultimately, this FOA is intended to support early stage testing of pharmacologic or device-based interventions using a protocol design where the presumed mechanism of action of the intervention is adequately tested, to provide meaningful information where target modulation yields a dose-dependent neurophysiological/clinical/behavioral effect.
- Early Stage Testing of Pharmacologic or Device -based Interventions for the Treatment of Mental Health Disorders (R33- Clinical Trial Required) – Funding Opportunity RFA-MH-18-703 from the NIH Guide for Grants and Contracts. The purpose of this Funding Opportunity Announcement (FOA) is to support the early stage testing of pharmacologic interventions with novel mechanisms of action or device-based interventions, for the treatment of symptoms or domains of altered functions in individuals with mental illness (e.g., schizophrenia, depression, autism, obsessive compulsive disorder, anxiety, bipolar disorder). Early intervention studies are also encouraged where symptoms of a disorder have been identified in subjects (a prodromal phase), prior to full diagnostic criteria being met. Ultimately, this FOA is intended to support early stage testing of pharmacologic or device-based interventions using a protocol design where the presumed mechanism of action of the intervention is adequately tested, to provide meaningful information where target modulation yields a dose-dependent neurophysiological/clinical/behavioral effect. Pediatric, adult and geriatric focused interventions are appropriate for this FOA. This R33 FOA supports single phased clinical trial awards. Applicants proposing high risk projects are encouraged to apply to the companion FOA, RFA-MH-18-702.
- Development of Psychosocial Therapeutic and Preventive Interventions for Mental Disorders (R61/R33- Clinical Trial Required) – Funding Opportunity RFA-MH-18-704 from the NIH Guide for Grants and Contracts. The purpose of this Funding Opportunity Announcement (FOA) is to support the efficient pilot testing of novel psychosocial therapeutic and preventive interventions for mental disorders in adults and children, using an experimental therapeutics approach. Under this FOA, trials must be designed so that results, whether positive or negative, will provide information of high scientific utility and will support “go/no-go” decisions about further development or testing of the intervention. This FOA supports the development and testing of innovative psychosocial intervention approaches where the target and/or the intervention strategy is novel. Targets might include, but are not limited to, potentially modifiable behavioral, cognitive, affective and/or interpersonal factors or processes, neural circuits or neural activity subserving specific behaviors or cognitive processes, and/or other neurobiological mechanisms associated with risk for, causation of, or maintenance of a mental disorder. Eligible psychosocial intervention strategies might include in-person or technology-assisted delivery, provided the target and/or the intervention strategy is novel. This FOA supports the development and testing of novel psychosocial interventions, as defined above, as monotherapies or as augmentations to standard treatment. Support will be provided for up to two years (R61 phase) for preliminary milestone-driven testing of the intervention’s impact on a target (a process or mechanism associated with risk for, causation, or maintenance of a clinical condition), that is, its target engagement. Contingent on meeting “go/no-go” milestones in the R61 phase, up to 3 years of additional support (R33 phase) may be provided for studies to replicate target engagement and relate change in the intervention target/mechanism to clinical benefit. Ultimately, this R61/R33 FOA is intended to speed the translation of emerging basic science findings of mechanisms and processes underlying mental disorders into novel interventions that can be efficiently tested for their promise in restoring function and reducing symptoms for those living with mental disorders, or for preventing mental disorders among those at risk.
|